plasmocytoma therapies
- Martin Döhring

- 28. Sept. 2025
- 3 Min. Lesezeit
Plasmacytoma is a type of cancer originating from plasma cells, which are differentiated B-cells responsible for antibody production. Its uniqueness at the molecular level stems from specific genetic, epigenetic, and molecular alterations that drive its malignant behavior. Below is a concise explanation of the key molecular characteristics of plasmacytoma cancer cells, particularly in the context of multiple myeloma (MM) or solitary plasmacytoma, based on current scientific understanding:
1. Genetic Alterations
Chromosomal Abnormalities:
Translocations: Plasmacytomas frequently exhibit immunoglobulin heavy chain (IgH) translocations, particularly involving chromosome 14q32. Common translocations include t(11;14)(q13;q32), which upregulates CCND1(cyclin D1), and t(4;14)(p16;q32), which dysregulates FGFR3 and MMSET. These translocations disrupt normal cell cycle regulation and promote uncontrolled proliferation.
Hyperdiploidy: Many plasmacytomas have extra copies of odd-numbered chromosomes (e.g., 3, 5, 7, 9, 11, 15, 19), leading to genomic instability.
Deletions and Mutations: Deletions in chromosome 13q (affecting RB1) and 17p (affecting TP53) are common and associated with poor prognosis. Mutations in oncogenes like KRAS, NRAS, and BRAF are also frequent, driving signaling pathways that promote cell survival and proliferation.
Clonal Evolution: Plasmacytoma cells exhibit clonal heterogeneity, with subclones acquiring additional mutations over time, contributing to disease progression and therapy resistance.
2. Dysregulated Pathways
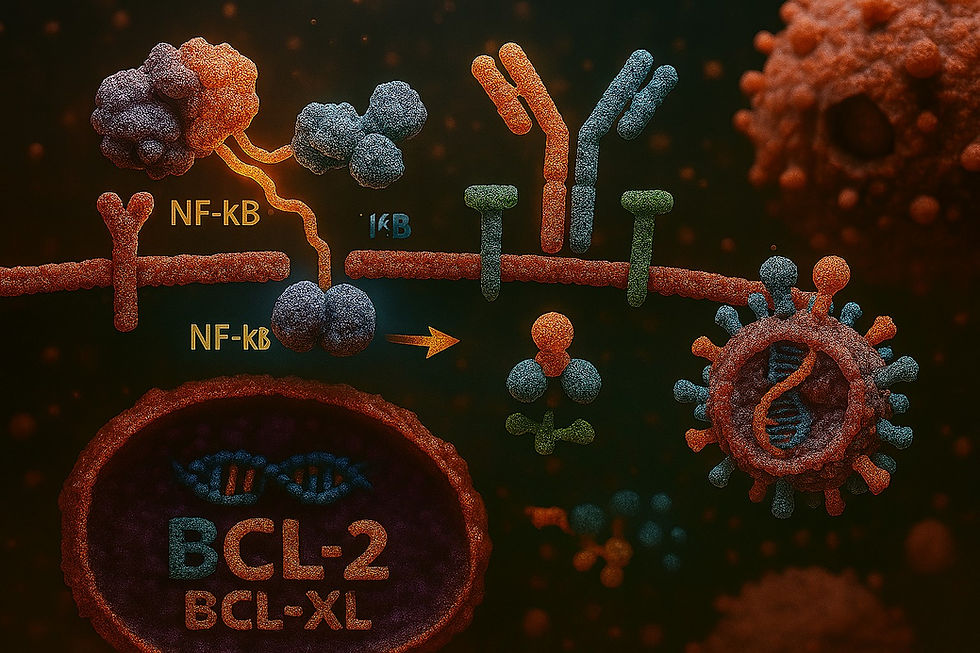
NF-κB Pathway: Constitutive activation of the NF-κB signaling pathway is a hallmark of plasmacytoma. This pathway promotes cell survival, proliferation, and resistance to apoptosis by upregulating anti-apoptotic genes (e.g., BCL-2, BCL-XL).
PI3K/AKT/mTOR Pathway: This pathway is often hyperactive, enhancing cell growth and survival in response to microenvironmental signals like IL-6 from bone marrow stromal cells.
MAPK Pathway: Mutations in RAS or BRAF lead to aberrant MAPK signaling, driving proliferation.
Cyclin D Dysregulation: Overexpression of cyclin D genes (CCND1, CCND2, or CCND3) due to translocations or other mechanisms disrupts the G1/S checkpoint, promoting uncontrolled cell division.
3. Epigenetic Modifications
DNA Methylation: Hypermethylation of tumor suppressor genes (e.g., p16, DAPK) silences their expression, contributing to oncogenesis.
Histone Modifications: Dysregulation of histone-modifying enzymes, such as MMSET (from t(4;14)), alters chromatin structure, leading to aberrant gene expression.
Non-coding RNAs: MicroRNAs (e.g., miR-21, miR-155) are dysregulated, influencing gene expression related to cell survival and proliferation.
4. Microenvironmental Interactions
Plasmacytoma cells are highly dependent on the bone marrow microenvironment. They overexpress adhesion molecules (e.g., integrins) and chemokine receptors (e.g., CXCR4), facilitating interactions with stromal cells that secrete growth factors like IL-6, VEGF, and IGF-1. These signals enhance tumor survival and drug resistance.
Overexpression of CD138(syndecan-1) on plasmacytoma cells supports adhesion and signaling within the bone marrow niche.
5. Protein Expression and Secretion
Monoclonal Protein Production: Plasmacytoma cells produce excessive monoclonal immunoglobulins (M-proteins) or light chains (e.g., kappa or lambda), detectable in blood or urine. This is due to their plasma cell origin and is a diagnostic hallmark.
Proteasome Dysregulation: Increased reliance on the ubiquitin-proteasome pathway for protein turnover makes plasmacytoma cells sensitive to proteasome inhibitors (e.g., bortezomib).
6. Unique Molecular Markers
CD38 and CD138: These surface markers are highly expressed and used for diagnostic and therapeutic targeting (e.g., daratumumab targets CD38).
SLAMF7: Overexpressed in plasmacytoma cells, making it a target for therapies like elotuzumab.
BCMA (B-cell Maturation Antigen): Highly expressed on malignant plasma cells, BCMA is a target for immunotherapies like CAR-T cells and bispecific antibodies.
7. Immune Evasion
Plasmacytoma cells downregulate MHC class I and co-stimulatory molecules, evading T-cell recognition.
They produce immunosuppressive cytokines (e.g., TGF-β, IL-10), creating a tumor-supportive microenvironment.
Clinical Relevance
These molecular features distinguish plasmacytoma from other cancers and drive its clinical behavior, including bone destruction (via RANKL overexpression), renal damage (from light chain deposition), and therapy resistance. They also inform targeted therapies, such as:
Proteasome inhibitors (e.g., bortezomib) for NF-κB and proteasome dysregulation.
Immunomodulatory drugs (e.g., lenalidomide) to disrupt microenvironment interactions.
Monoclonal antibodies (e.g., daratumumab, elotuzumab) targeting CD38 and SLAMF7.
BCMA-targeted therapies for advanced disease.
Sources
The information is based on general knowledge of plasmacytoma and multiple myeloma molecular biology, consistent with scientific literature up to September 2025. For specific details, studies from journals like Blood, Nature Reviews Cancer, and Journal of Hematology & Oncology provide comprehensive insights into plasmacytoma’s molecular profile.








Additional Molecular Nuances
MYC Dysregulation
Later in disease progression, plasmacytomas often acquire MYC translocations or amplifications, which drive aggressive growth and clonal evolution. MYC upregulation is considered a “late event” distinguishing indolent MGUS (monoclonal gammopathy of undetermined significance) from malignant plasmacytoma/MM.
DNA Damage and Repair Pathways
Many plasmacytomas show defective DNA damage response (DDR), particularly involving ATM/ATR signaling. This makes cells more reliant on error-prone repair mechanisms, fueling genomic instability and creating therapeutic vulnerabilities (e.g., PARP inhibition in selected cases).
Immune Checkpoints in Evasion
Beyond reduced MHC expression, plasmacytoma cells upregulate PD-L1 (programmed death-ligand 1), further suppressing T-cell activity. This is an active area of therapeutic investigation.
Bone Disease Link (Osteolysis)
Plasmacytomas secrete factors that tilt the balance toward osteoclast activation and…